Development
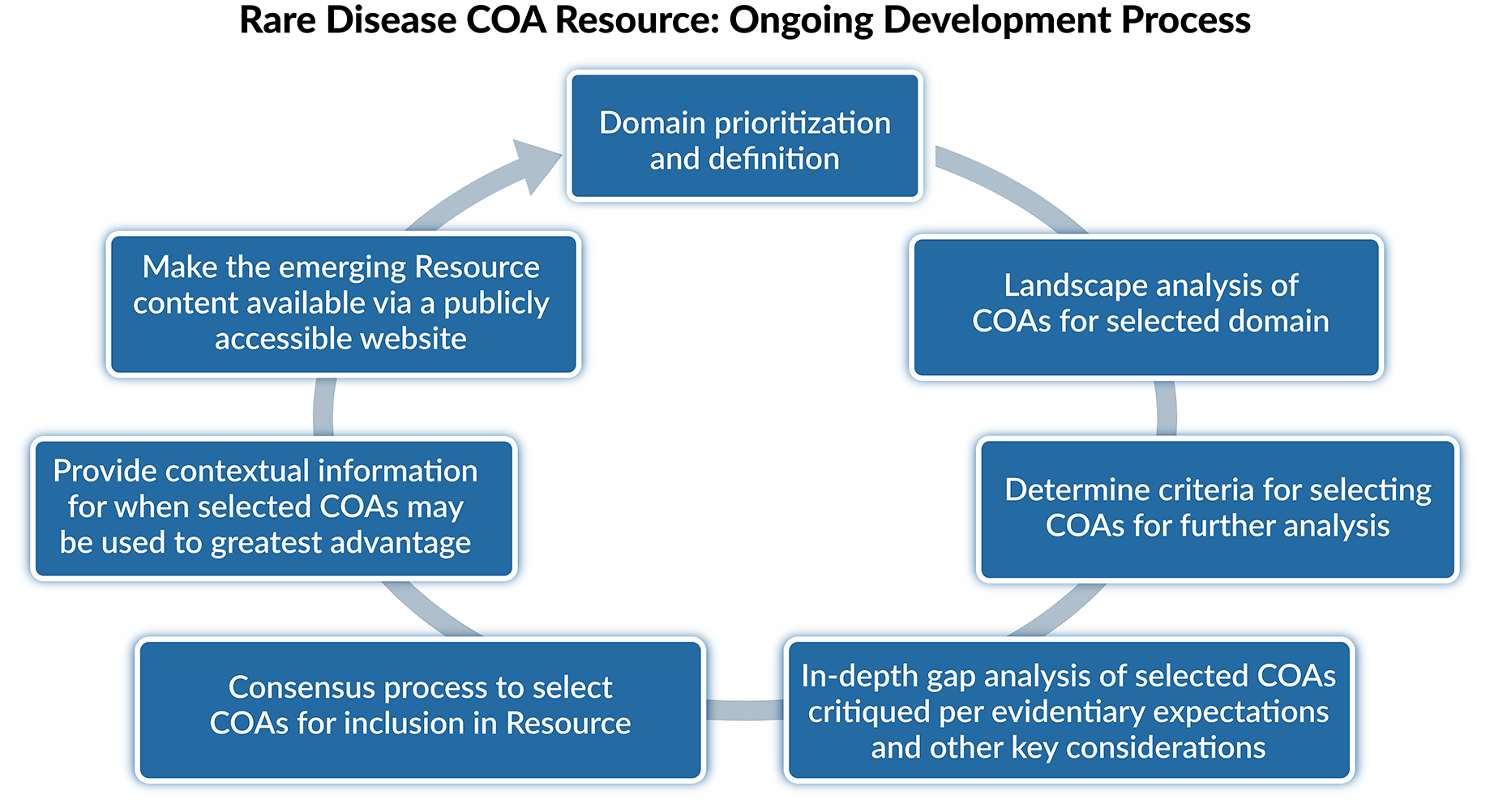
A domain-level approach was selected to begin populating the Rare Disease COA Resource. The first iteration of the Rare Disease COA Resource focuses on the assessment of daily function in pediatric, non-oncologic populations. This overarching domain was broken down into the daily function subdomains of self-care, gross motor function, fine motor function, and communication. Other subdomains will be added over time.
Development
A domain-level approach was selected to begin populating the Rare Disease COA Resource. The first iteration of the Rare Disease COA Resource focuses on the assessment of daily function in pediatric, non-oncologic populations. This overarching domain was broken down into the daily function subdomains of self-care, gross motor function, fine motor function, and communication. The second iteration of the Resource, launched in December 2025, was expanded to include pain behavior, pain severity, sleep disturbance, and sleep impact. Other subdomains will be added over time.
Inclusion of COAs in the Resource
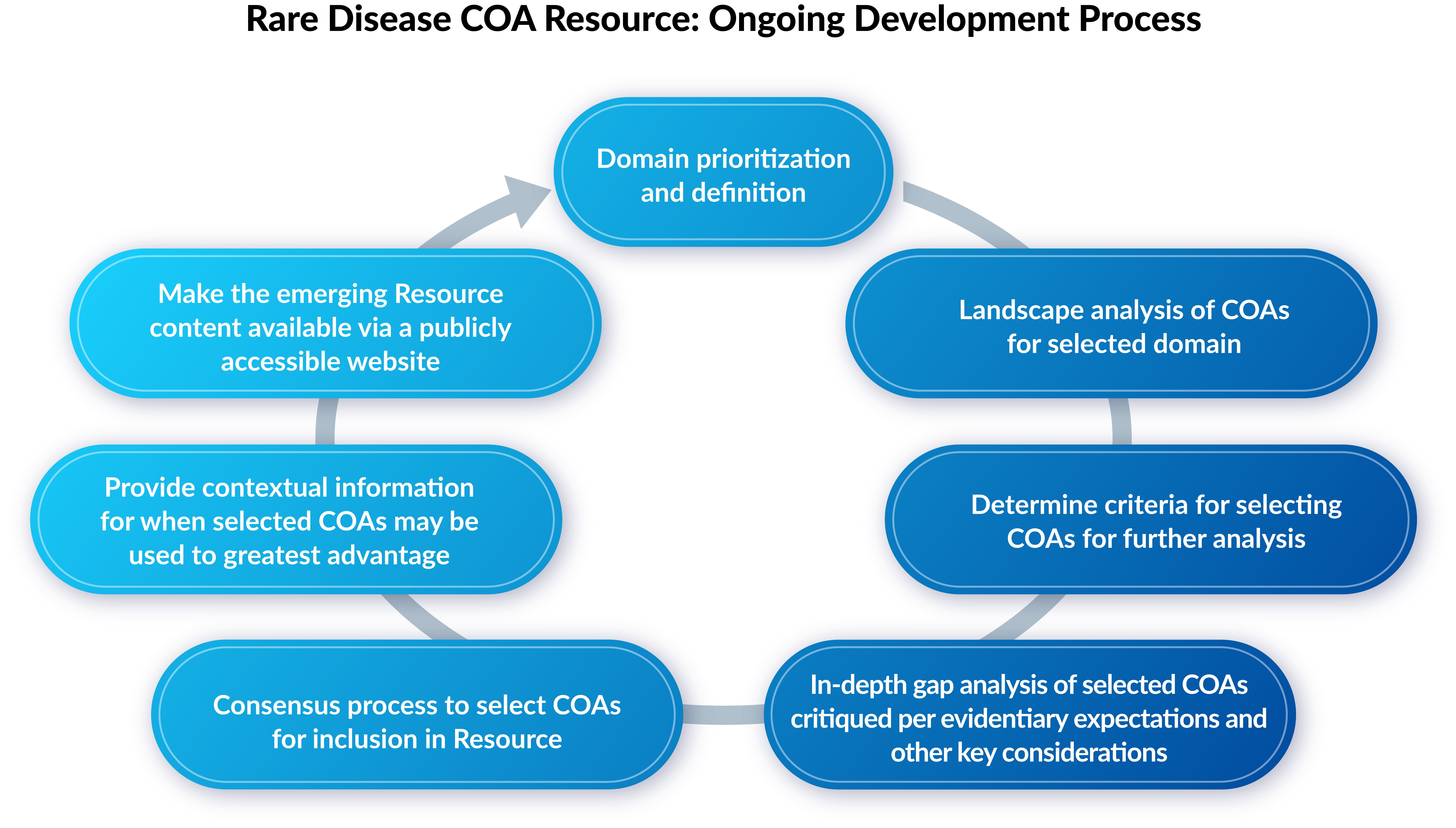
A Rare Disease COA Resource Development Subcommittee was formed to select COAs for inclusion in the Rare Disease COA Resource. Members of this subcommittee included representatives from sponsor firms with expertise in the domains of interest, FDA colleagues, academic partners with clinical expertise, and COA development experts. Advisory Panels were convened to review the results of gap analyses and selected COAs. Panel membership for the daily function subdomains of self-care, gross motor function, and fine motor function consisted of an Occupational Therapist, Physical Therapist, Caregiver Representative, and for Communication/Language an Occupational Therapist, Speech and Language Pathologist, and Caregiver Representative. Panel membership for the pain subdomains of pain behavior and pain severity, and sleep subdomains of sleep disturbance and sleep impact consisted of 2 caregivers representing Rett Syndrome and GM1 gangliosidosis as well as 1 person with lived experience with Duchenne Muscular Dystrophy.
A consensus process between each Advisory Panel and the Rare Disease COA Resource Development Subcommittee determined the final COAs to include in the Rare Disease COA Resource.
FDA funding disclosure
Critical Path Institute is supported by the Food and Drug Administration (FDA) of the Department of Health and Human Services (HHS) and is 43% funded by the FDA/HHS, totaling $20,724,703, and 57% funded by non-government source(s), totaling $27,346,613. The contents are those of the author(s) and do not necessarily represent the official views of, nor an endorsement by, FDA/HHS or the U.S. Government.
Funding for the establishment of the Rare Disease COA Consortium was made possible, in part, by the Food and Drug Administration through grant (U01FD006).
